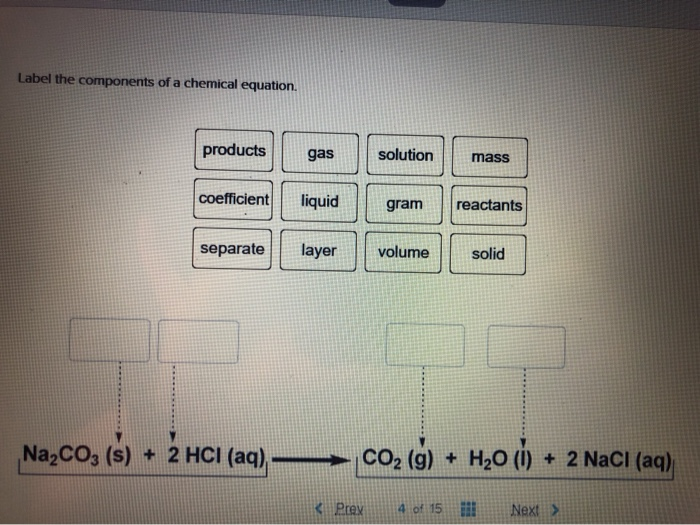
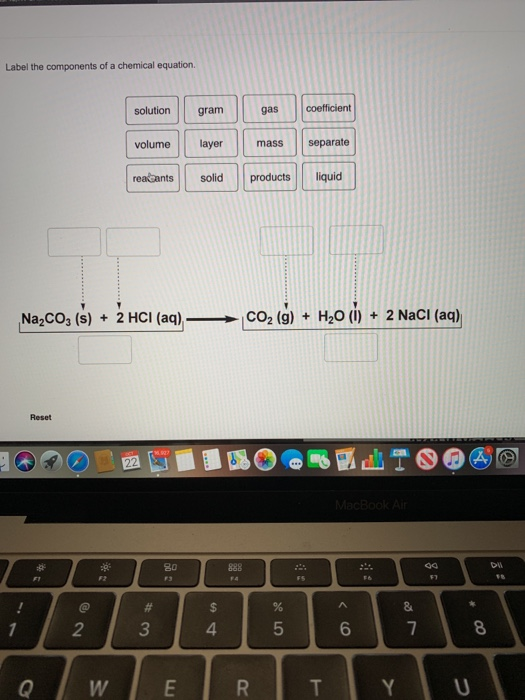
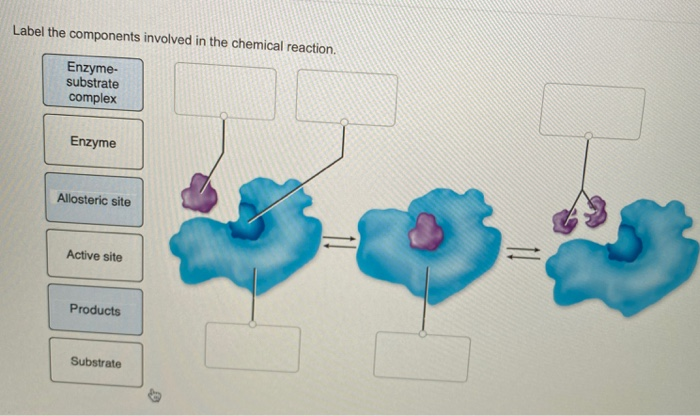
42 label the components of a chemical equation.
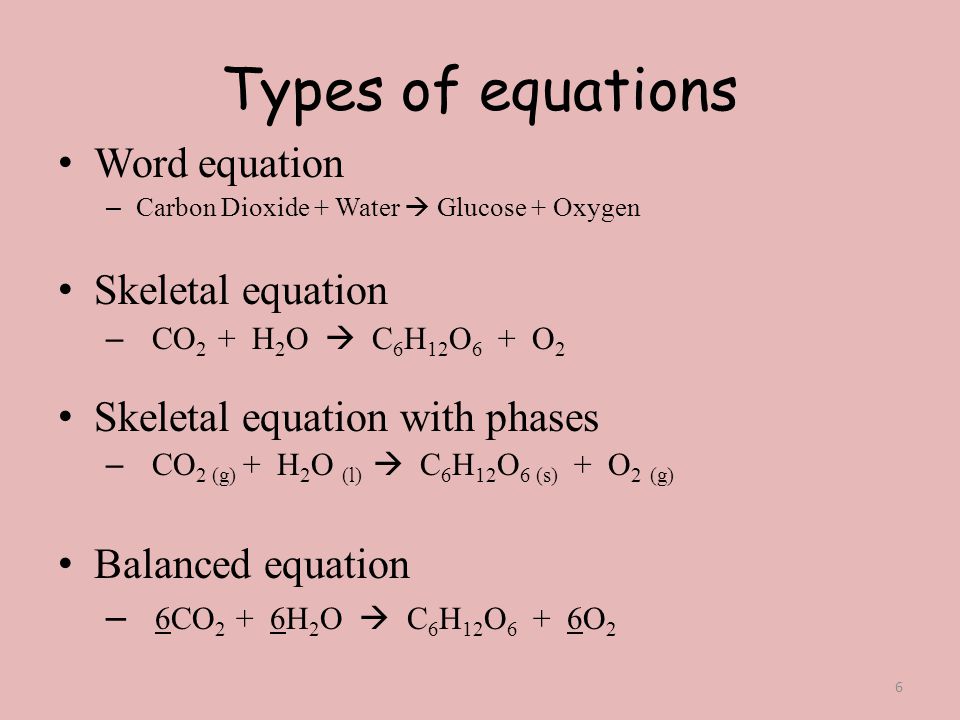
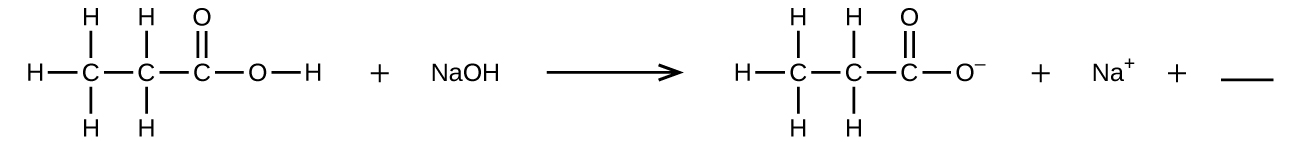
4.1 Writing and Balancing Chemical Equations - Chemistry Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO2(aq)+NaOH(aq) Na2CO3(aq)+H2O(l) (unbalanced) CO 2 ( a q) + NaOH ( a q) Na 2 CO 3 ( a q) + H 2 O ( l) ( unbalanced) What is the chemical equation for cellular respiration? | Socratic The overall (unbalanced) chemical equation for cellular respiration is: "C"_6"H"_12"O"_6 + "O"_2 → "CO"_2 + "H"_2"O" + "energy" > The balanced equation is "C"_6"H"_12"O"_6 + "6O"_2 → "6CO"_2 + "6H"_2"O" + "energy" The equation expressed in words would be: "glucose + oxygen → carbon dioxide + water + energy" The equation is formulated by combining the three following processes into one ...
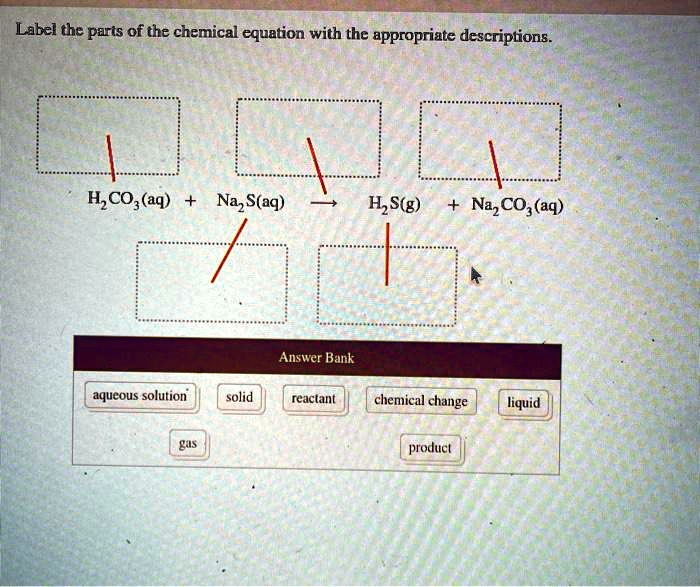
Solved Label the components of a chemical equation. solution | Chegg.com Chemistry Chemistry questions and answers Label the components of a chemical equation. solution gram gas coefficient volume layer mass separate realants solid products liquid Na2CO3 ($) + 2 HCI (aq) —>CO2 (g) + H20 (1) + 2 NaCl (aq)
:max_bytes(150000):strip_icc()/aceticacid-56a129995f9b58b7d0bca2c4.jpg)
Label the components of a chemical equation.
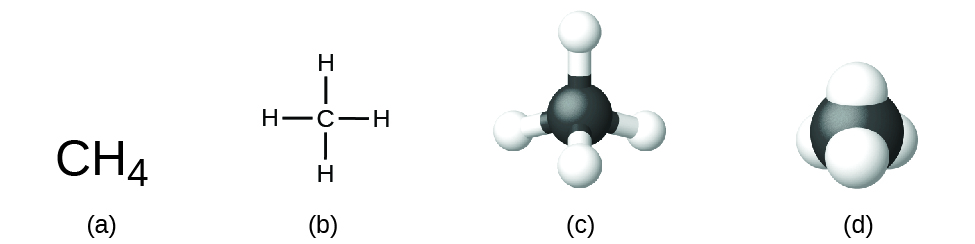
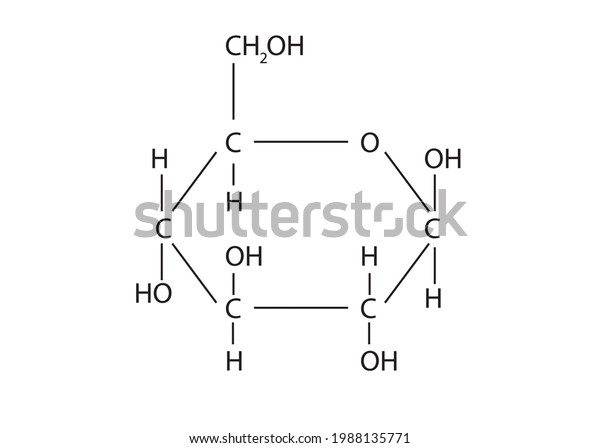
Chemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction. Set 1 - Parts of a Chemical Equation Diagram | Quizlet A number in front of a chemical formula in an equation that indicates how many molecules or atoms of each reactant and product are involved in a reaction. Subscript A number in a chemical formula that tells the number of atoms in a molecule or the ratio of elements in a compound yield to produce Reactants Chemical Nomenclature and Chemical Formulas - Owlcation It consists of symbols of elements and subscripts which give the number of atoms of each element. Examples: The formula of water is H2O There are 2 atoms of Hydrogen and 1 atom of oxygen The formula of glucose is C6H12O6 There are 6 atoms of Carbon, 12 atoms of Hydrogen and 6 atoms of Oxygen.
Label the components of a chemical equation.. Chemical Nomenclature - Chemistry 2e Derive names for common types of inorganic compounds using a systematic approach. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4. What Is the Chemical Equation for Cellular Respiration? Chemical equations use chemical formulas in a visual representation of the reaction. Chemical formulas represent a molecule with element symbols and numbers. C represents carbon, H represents... What is a Chemical Equation? - Definition & Examples Atoms & Molecules in an Equation As you've learned, the main parts of a chemical equation are the reactants and the products. Besides these, however, there are other pieces of information... How to Write a Chemical Equation (with Pictures) - wikiHow In a basic double replacement equation you will have 2 cations and 2 anions. The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2
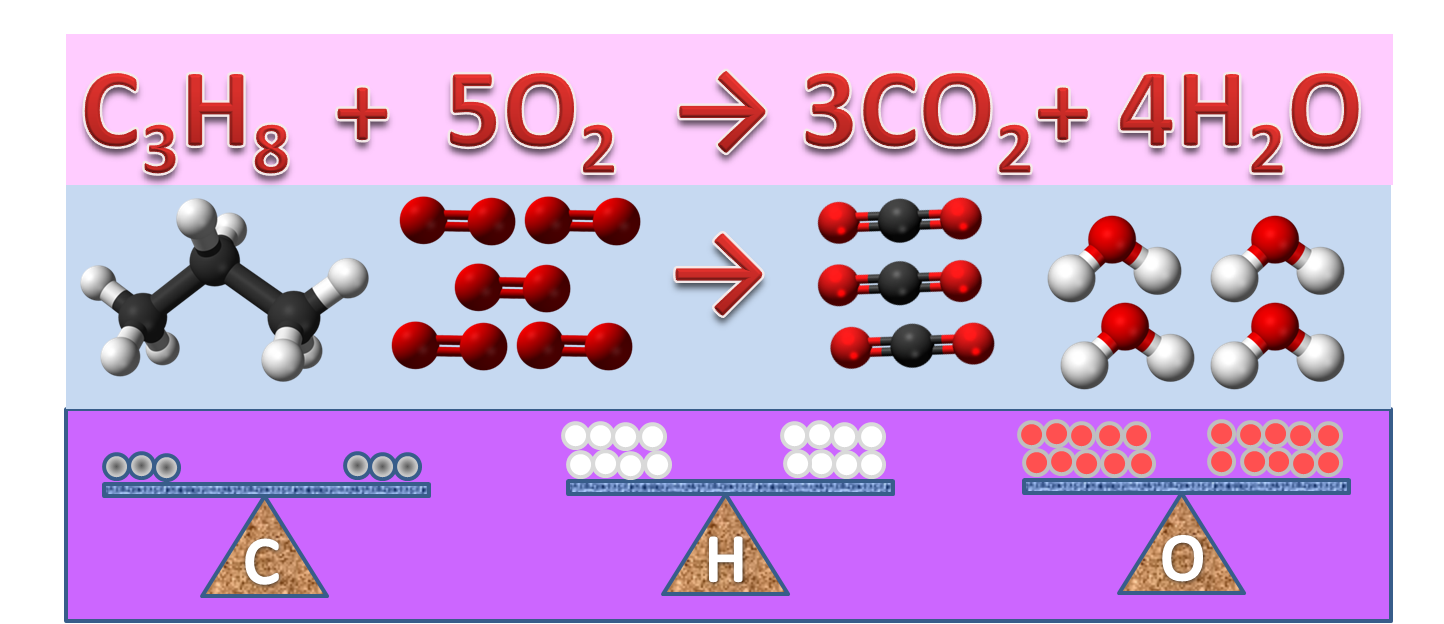
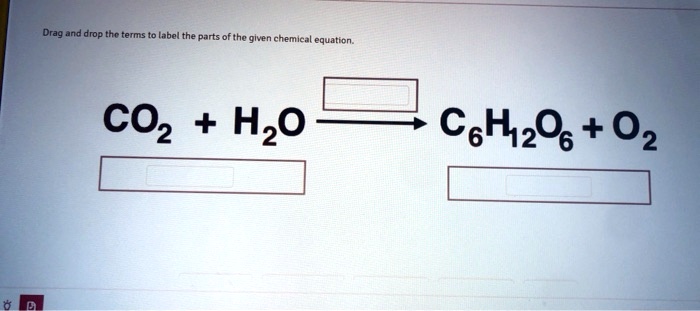
What Are Chemical Equations? - ThoughtCo It's common to indicate the state of matter in a chemical equation by including parentheses and an abbreviation right after a chemical formula. This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. The Chemical Equation For Photosynthesis | Science Trends The balanced chemical equation for photosynthesis is as follows: 6 CO2 + 6 H2O → C6H12O6 + 6 O2 This translates to the production of glucose and oxygen from carbon dioxide and water. O2 Is known as dioxygen but frequently referred to as simply oxygen. 4.2 Classifying Chemical Reactions - Chemistry Writing Equations for Acid-Base Reactions Write balanced chemical equations for the acid-base reactions described here: (a) the weak acid hydrogen hypochlorite reacts with water (b) a solution of barium hydroxide is neutralized with a solution of nitric acid. Solution (a) The two reactants are provided, HOCl and H 2 O. Chemical Equation | Reactants And Products In Chemical Reactions The representation of a chemical reaction in the form of symbols (substances) is known as chemical equation. A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation.
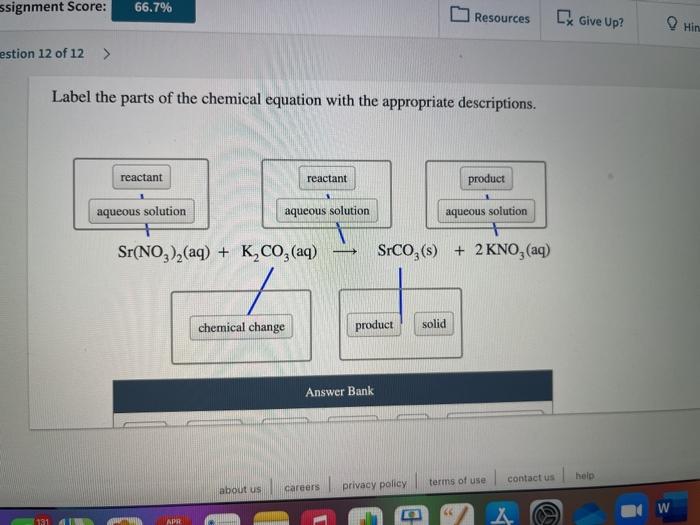
Solved Label the components of a chemical equation. separate | Chegg.com Chemistry Chemistry questions and answers Label the components of a chemical equation. separate mass liquid coefficient gas solution solid reactants layer prod uiets volume gram Na2co, (s) + 2HCl (aq),- ?.co2 (g) + H2O (l) Co2 (g) + H20 ) + 2 Naci (aq) Examples of Balanced Chemical Equations - ThoughtCo A balanced equation contains the same number of each type of atoms on both the left and right sides of the reaction arrow. To write a balanced equation, the reactants go on the left side of the arrow, while the products go on the right side of the arrow. Coefficients (number in front of a chemical formula) indicate moles of a compound. Chemical Formulas in Food - Explained - FoodCrumbles Examples of chemical formulas in food. To get more familiar with the concept of chemical formulas, here are some more very common food molecules written as a chemical formula: Glucose - C 6 H 12 O 6. Sucrose (standard sugar) - C 12 H 22 O 11. Lactose - C 12 H 22 O 11. Linoleic acid (a fatty acid) - C 18 H 32 O 2. What are Chemical Equations? Detailed Explanation, Examples The chemical formulae for all the elements that form each molecule and uses a small number to the bottom right of an element's symbol to stand for the number of atoms of that element. For example, the chemical formula for water is H2O. Why is it crucial to balance a chemical equation?
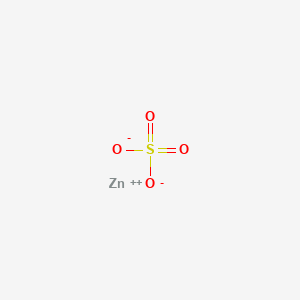
Chemical formula - Wikipedia A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs.
Symbols in Chemical Equations - Harper College Symbols in Chemical Equations. Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read used when the reaction can proceed in both directions - this is called an equilibrium arrow and will be used later in the ...
Chemical Ingredients 101: How to Read a Product Label Calcium carbonate, dehydrated silica gels, hydrated aluminum oxides, magnesium carbonate, phosphate salts and silicates aid in the removal of tooth debris and residual surface stains. 1 Sorbitol, a type of sugar derived from fruits, corn and seaweed, helps improve the taste of toothpaste. 2 An example of a toothpaste label
Chemical Equations Flashcards | Quizlet Chemical Equation symbolic representation of a chemical reaction, will show the same # of each type of atom on each side of the equation. ex. Na + Cl2 -> NaCl Chemical Formula symbolic representation of of an element or compound. ex. NaCl (table salt) Chemical Reaction process in which bonds between atoms are broken and new bonds are formed.
Parts of a Chemical Equation | Chemistry Quiz - Quizizz answer choices Describes the number of elements in a compound. One or two letters that represent the elements on the periodic table. The number to the lower right of an element that shows the number of atoms bonded. A written description of a chemical reaction. Report Quiz
Parts of a Chemical Equation Worksheet | Aurumscience.com. Parts of a Chemical Equation All of the basic parts of a chemical reaction are covered by this worksheet. Students will identify the reactants, products, subscripts, and coefficients. Included is information on the state of matter notation that indicates whether each substance is a solid, liquid, gas, or aqueous solution.
Capitalization of Chemical Compounds | AJE Chemical formulas. Within a sentence, the names of chemical compounds are not capitalized, but the first letter of each elemental symbol should be capitalized (e.g., "We added sodium hydroxide " and "We added NaOH ). Note that symbols and words should not be mixed (that is, avoid saying " K chloride "). 4. Amino acids.
Balancing chemical equations (how to walkthrough) (video) - Khan Academy N2 + H2 -> NH3. On the left there is 2 N and 2 H. On the right there is 1 N and 3 H. If we tried to balance starting with H you'd need to use a fraction or decimal and would get messy, so let's start with N. There's 2 on the left and 1 on the right, so we need to change the coefficient of NH3 to 2.
3 Steps for Balancing Chemical Equations - ThoughtCo 3 Steps for Balancing Chemical Equations 1) Write the unbalanced equation. Chemical formulas of reactants are listed on the lefthand side of the equation. Products are listed on the righthand side of the equation. Reactants and products are separated by putting an arrow between them to show the direction of the reaction.
What are the Parts of a Chemical Equation? - Life Persona Basically there are three Main parts in a chemical equation : The reactants, the products and the arrow indicating the direction of the chemical reaction. A chemical equation is an abbreviated form of representing the components of a chemical reaction.
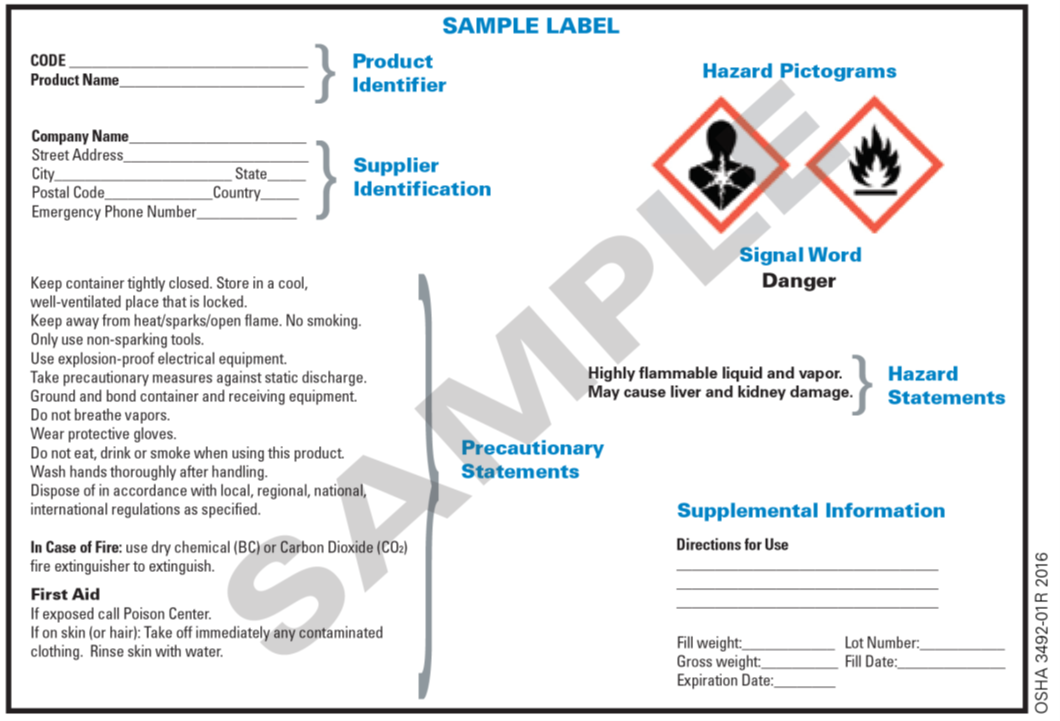
What are the 6 Elements of a GHS Label? - Computype Pictograms 1. Product Identifier/Ingredient Disclosure This component of the GHS label is typically placed in the top left-hand corner of the label, and it identifies the hazardous chemical or ingredient that is in this product. It can state the name, code number, or batch number. This allows for the chemical to be confidently identified. 2.
Chemical Nomenclature and Chemical Formulas - Owlcation It consists of symbols of elements and subscripts which give the number of atoms of each element. Examples: The formula of water is H2O There are 2 atoms of Hydrogen and 1 atom of oxygen The formula of glucose is C6H12O6 There are 6 atoms of Carbon, 12 atoms of Hydrogen and 6 atoms of Oxygen.
Set 1 - Parts of a Chemical Equation Diagram | Quizlet A number in front of a chemical formula in an equation that indicates how many molecules or atoms of each reactant and product are involved in a reaction. Subscript A number in a chemical formula that tells the number of atoms in a molecule or the ratio of elements in a compound yield to produce Reactants
Chemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction.
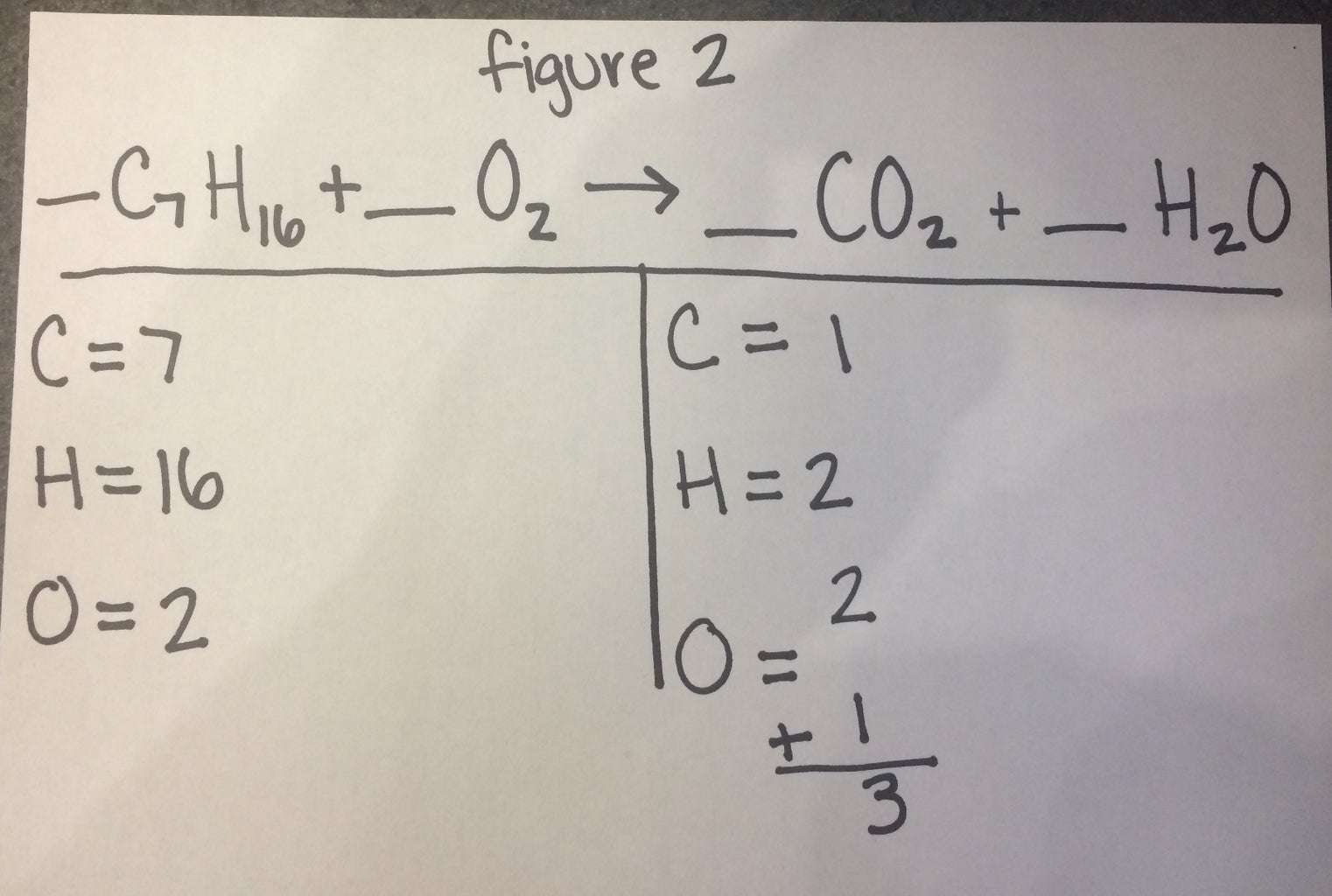









:max_bytes(150000):strip_icc()/aceticacid-56a129995f9b58b7d0bca2c4.jpg)
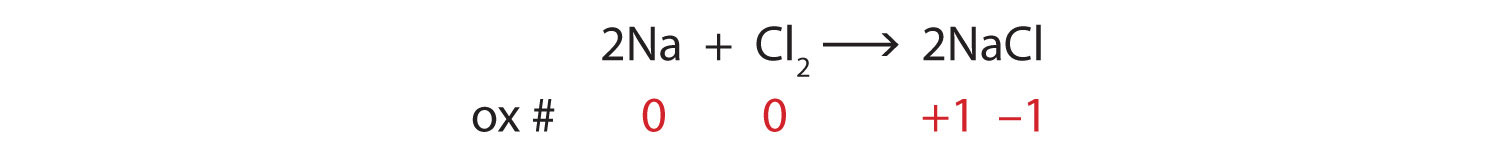



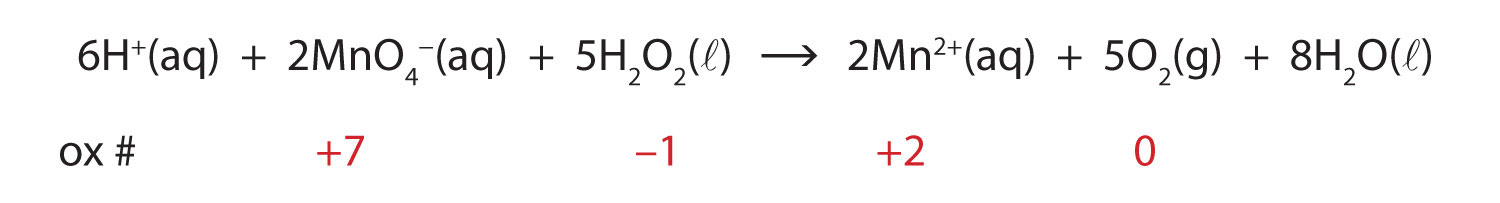





Post a Comment for "42 label the components of a chemical equation."